Group of Optical and Magnetic Properties of Solids (GPOMS)
- Home
- Research
- DEQ - Department of Quantum Electronics
- Group of Optical and Magnetic Properties of Solids (GPOMS)
The Group of Optical and Magnetic Properties of Solids (GPOMS) performs experimental research on solid substances with unique properties, such as superconductors, nanostructures (objects with "details" of a few atoms in size), materials with outstanding magnetic properties and substances that present the so-called "quantum phase transitions." Part of the research is done in several leading edge equipments located within the group itself, while part is done at large open laboratories in Brazil and abroad, such as the National Synchrotron Light Laboratory (LNLS), NIST Center for Neutron Research (NCNR), National High Magnetic Field Laboratory (NHMFL) and Argonne National Laboratory (ANL).
The group keeps pursuing new materials with extraordinary properties. Currently, GPOMS is almost independent on the production of materials used in much of its research and has the ability to perform a range of experiments to better understand the physical properties of these materials. With this motivation, GPOMS is always looking for new materials design, technological upgrades of the existing ones and/or improving the scientific knowledge about their properties. Some physical phenomena such as superconductivity at high critical temperatures and their relationship with magnetism are not fully understood yet. The group's contributions aim to improve the fundamental understanding of these phenomena, both through experiments and through unpublished theoretical investigations.
The experimental techniques used in the investigation of materials are mainly: Electron Paramagnetic Resonance, Raman Spectroscopy, Magnetometry, Electrical resistivity, specific heat, X-ray absorption and diffraction, neutron scattering, and soon nuclear magnetic resonance and nuclear quadrupole resonance.
There is much talk about superconductors and its potential to revolutionize technology, since its main feature is not wasting any energy when conducting electricity - unlike ordinary conductors, in which part of the energy is lost as heat. However, due to some practical difficulties, they have not flooded the market as one would expect given its great technological potential. The reason is that there is a Gordian knot in research about them: they either become superconductors at temperatures so low that make them prohibitively expensive, or they are ceramic, brittle, with very low critical currents, which limits its wide use in our day- to-day. The current problem then is reduced to how to make malleable superconductors (metallic) while acquiring superconductivity at temperatures sufficiently high to be commercially exploitable.
Many scientists believe the solution may be found in certain substances which are metallic but in spite of becoming superconducting only below -233 degrees Celsius (therefore remaining expensive), have many properties in common with high critical temperature (the temperature at which the material starts to exhibit superconductivity). If the reason why they possess these properties is understood, it may be possible to discover how to make a superconducting metal at temperatures compatible with commercial viability.
This is one of the most important research lines of GPOMS today.
For example, there are the so-called heavy fermion superconductors. In these materials, the electrons that conduct electricity do it in groups, which behave as "quasi-particles" with hundreds of times the individual electron mass. Therefore, these materials are called "heavy fermions" (fermion is a class of elementary particles to which the electrons belong).
Of particular interest are the heavy fermion compounds of cerium, indium and one of the so-called "transition metals", such as cobalt or rhodium (these are compounds such as "cerium-m-indium-5" where "m" is the transition metal). They are useful for reproducing very well the basic characteristics of the high critical temperature superconductors (HTC), despite its very low critical temperature. Moreover, it is possible to sinthesize crystalline samples of great quality, and the properties of the HTC they reproduce are "pure", without additional complications. This makes this family an ideal instrument to investigate why these materials look so much like the HTC superconductors without being them.
On the other hand, the cerium-m-indium-5 also presents another feature that puts it in the frontline of research about superconductors. It presents, at the same time, superconductivity and antiferromagnetism. This is interesting because one still does not quite understand the correlation between these two properties. The theories on superconductivity do not explain well how this can happen. The actual explanation may lead to important news about superconductors or even about other areas of physics.
Certain systems of ceramic crystals such as the double perovskites (Figure below) have been thoroughly studied because of their unique properties, such as colossal magnetoresistance and other electric and magnetic properties that seem to defy theoretical understanding.
Magnetoresistance is a variation in the electrical resistance of a material when an external magnetic field is applied. That is, when placed within a field, it can more easily conduct electricity. This effect is well known and explained. However, in general, this variation is less than 5%. However, in 1993, were discovered material whose electrical resistance decreases to one hundredth, a thousandth or less.
The perovskites also have special properties related to ferromagnetism (characteristic property of a magnet) and ferroelectricity (analogous to ferromagnetism, except for that a spontaneous electric rather than magnetic field is generated). They may also present related phenomena such as antiferromagnetism – in which the small magnetic units that align to produce the magnetic field are in alternating directions, coupled with ferroelectricity. These electrical and magnetic properties, as well as the possible coupling between them, have great potential to push the next generations of electronic devices.
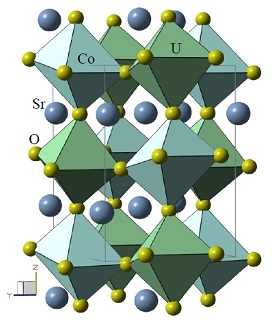
Scheme of the crystal structure of the double perovskite Sr2CoUO6. This unit is repeated throughout the volume of the material. It is formed by atoms of strontium (Sr), cobalt (Co), uranium (U) and oxygen (O). The green octahedra have a cobalt and the blue ones have a uranium ion in the center. These octahedra are formed by oxygen atoms (in their vertices)
Source: Doctoral thesis of Ali Francisco García Flores, IFGW / Unicamp (2007), p. 58
"Nanomaterials", the new great promise of modern technology, are objects with tiny structure ("details"), about the size of a few tens of atoms. Interestingly, when an object has this size, it may exhibit different properties from those of normal size materials with identical composition. The reason is that the surface of a common material has properties that are different from its interior. For example, the water surface has a surface tension capable of maintaining, without sinking, a mosquito or even a carefully placed clips. But when the quantity of material is very small, the surface properties interfere with its interior and the result is something with quite different characteristics. A large branch of physics today is dedicated to investigate new properties which may have nanosized materials and what you can do with them.
In the case of GPOMS, research in nanomaterials is mainly done with Magnetic Resonance. The idea is to investigate how the nanostructures behave in extreme conditions: extremely cold (below 50 thousandths of a degree above absolute zero - the "absolute zero" is the lowest possible temperature of -273.15 degrees Celsius); very intense magnetic fields (14 teslas or about 1500 times the common field of a magnet); very large pressure (30 kbar, or the equivalent of 30 thousand kilograms rested on a surface of one square centimeter).
Another line of investigation, in a more fundamental level, is about the quantum phase transitions. Well known examples of phase transitions are liquefaction, solidification, boiling, fusion, etc. These occur with varying temperature. There are, however, transitions appearing with variations of other parameters, such as pressure, magnetic field, or even subtle changes in chemical composition of the substance (so-called "doping"). Some of these are classified as "quantum phase transitions" and happen near the absolute zero temperature, a situation in which the electrons may start to display coupled behavior - so the situation is called "high electronic correlation." These are widely studied phenomena because such collective behaviors often show unusual "emerging" features and are related to fundamental aspects of quantum physics and its interface with classical physics.
The group designs materials that exhibit quantum phase transitions and characterizes them, trying to understand this phenomenon and works to improve techniques to obtain new substances with this property.
There is a connection between this line of research and superconductivity. Many scientists believe that the study of the relationship between magnetism and quantum phase transitions will throw light on the causes of superconductivity at high critical temperatures. When these causes are well understood, it will be easier to design superconductors with higher and higher critical temperatures.
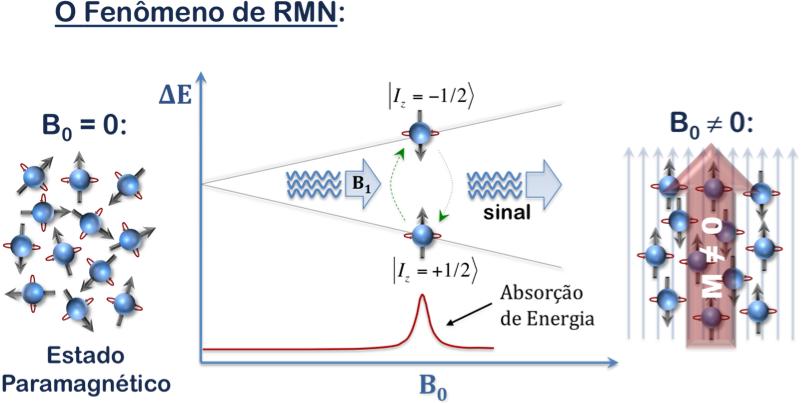
There are two types of Magnetic Resonance: the Electronic or Electronic Spin (EPR) and nuclear (NMR).
NMR is a physical phenomenon exhibited by the nuclei of certain chemical elements that, when subjected to a strong magnetic field and excited by radio waves (fr) at a specific frequency (known as the Larmor frequency), absorb energy from the system, and then emit a radio signal which can be picked up by an antenna and transformed into spectroscopic signal, or even an image. The same principle is valid to EPR, however for electrons instead of nuclei and in another frequency range.
This technique is well known in medicine for being non-invasive and widely used to produce internal images of the human body (MRI: Magnetic Resonance Imaging) and enables accurate diagnosis for various diseases. Within the area of interest of our group, it is considered a very powerful microscopic technique that provides detailed information on the crystalline (and molecular) structure, on the dynamics of atoms and electrons, the states of reaction, phase transitions, and chemical vicinity of molecules and crystalline materials.
In this technique, the object under investigation is placed in two magnetic fields, one of them steady and the other oscillating. In the case of nuclear magnetic resonance, these fields make the nuclei of atoms behave like tops precessing around the direction of the constant external field (see figures below). With this movement, the atoms emit electromagnetic waves that carry information about the material. The tomography devices analyze these electromagnetic waves and make images of the human body. Therefore, it is also called "Magnetic Resonance Imaging (MRI)"
In the case of electron spin resonance, the principle is the same, except that the electrons instead of the nuclei of atoms spin like spinning tops around the field

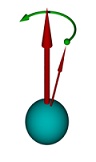
A spinning top has two movements: a fast one, around itself, and the "precession", in which its inclination slowly rotates around the vertical. In the above picture, to the right, the thicker arrow indicates the direction of the fixed magnetic field, the thinner arrow shows the direction around which the electron "spins" around himself, the green curved arrow indicates the movement of precession, just like a spinning top
Source of the figures: Wikipedia
In this method, a laser is directed onto the object one wants to investigate and the scattered light is analyzed. The frequency (ie, the color) of the scattered light may be different from that of the incident laser because the light can transfer some of its energy to the material studied, or even receive some energy. This is the so-called "Raman effect". Analyzing these changes in the scattered light, one can infer various characteristics of the material under investigation.
Tiny objects such as molecules, atoms and subatomic particles, generally cannot have any arbitrary energy, but only certain specific values. Energy is composed of units, packets called quanta (plural of quantum, by the Latin spelling). It's like "atoms" of energy, with the difference that the atoms of the same substance are all the same, while the size of quanta is not fixed but depends on the situation. Thus, the material - any material: a lamp filament, the sun etc. - Can only emit or absorb energy in quanta integers.
It turns out that the set of allowed energies is a feature, a "signature" of each atom, molecule etc. Spectroscopy is just a technique that gives access to these energies. It determines the spectrum, ie, the set of specific energies which the material can emit, absorb or scatter. With that, one can identify the composition of things - from new and unknown materials to astronomical objects such as stars.
To determine the spectrum of the emitted, absorbed or scattered light by a material, just separate the light into its colors, like the sunlight in rainbow or how we can make a prism
Source of figure: Wikipedia.
In the case of Raman spectroscopy, one throws light of a certain color (frequency) onto the object. Much of the scattered light by the material has the same color of the incident light: this is called Rayleigh scattering. However, a small fraction is scattered with a different color, and the difference in frequency of the incident and scattered light is proportional to the gain or loss of energy due to the light interactions with the material. This is the Raman scattering. Now, it is the set of changes in the frequencies of the scattered light (the spectrum) that form the "signature" of the studied material that allows one to identify it; and it also allows us to measure their various microscopic characteristics, such as the strength of the chemical bonds between its atoms and molecules, electronic and magnetic excitations, magnetic, among others.
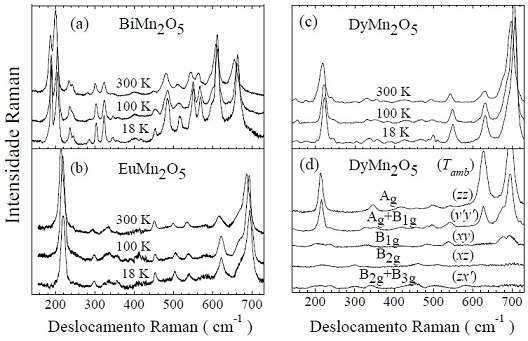
Aspect of the graphs obtained with Raman spectroscopy for four different substances. The "peaks" of the curves are due to specific vibrations of atoms, which have well-defined energies; they are different for each substance and constitute a "signature" of it. For each of the first three figures, there are three curves, each for a distinct temperaturee (300 K or 27 oC; -173 o C = 100 K, and 18 K = -255oC). In graph (d), each of the five curves was done with the incident and scattered light with different polarizations
Source: PhD thesis of Alí Francisco García Flores, IFGW/Unicamp (2007), pág. 14
About them, we are all familiar with the images that doctors usually order. However, GPOMS uses these beams in other, quite different ways.
One of these techniques is X-ray diffraction. It is most commonly used to study crystalline materials, i.e. materials in which the atoms are arranged in a certain order that is repeated along the three directions (if not, the material is called "amorphous"). When X-rays go through a crystalline material, they are deflected by atoms (technically, "diffracted" by the material) in some directions and with specific intensities. This forms regular patterns (because of the regularity of atoms) called diffraction patterns. By analyzing these patterns, one can find out what are the relative positions of the atoms in the material.
X-rays are mostly deflected by the electric charge of the electons inside the atoms themselves, also being sensitive to their magnetic moments (the electrons behave as tiny magnets - when, in fact, many of them line up in the same direction, their tiny magnetic fields add up leading to the ordinary magnets). One can also analyze diffraction patterns generated by the magnetism of the electrons, obtaining information about the magnetic structure of the material of interest.
Besides diffraction, X-rays can also be used in other ways: for instance, by exploiting its absorption by the material of interest. This provides information, complementary to diffraction, on the arrangement of atoms, the nature of the internal magnetic fields, the occupation of electrons in each atom (ionicity), among others. It's called X-ray Absorption Spectroscopy (XAS), which encompasses a set of techniques, such as EXAFS (acronym for "Extended X-ray Absorption Fine Structure"), XANES ("X-ray Absorption Near Edge Structure") and XMCD ("X-ray Magnetic Circular Dichroism"). These techniques differ from each other in certain technical details about how the absorption of X-rays is measured and analyzed. Choosing appropriately the frequency of the X-rays, one may obtain specific information about each element that composes the material separately. Moreover, the material needs not to be ordered (crystalline) to be studied by XAS echniques, contrary to X-ray diffraction – indeed, they are complementary techniques.
How are the X-rays, which serve as "probes" of the inner structure of the material, produced ? The group uses the Brazilian Synchrotron Light Laboratory (LNLS). This is an electron accelerator that makes them travel at speeds very close to that of light, turning in "circles" (actually a 12-sided polygon). When they are forced by the magnetic fields to follow a curved path, the electrons emit electromagnetic waves at many frequencies ("colors"), including infrared, ultraviolet, X-rays and visible light. This radiation is called "synchrotron radiation" and is very useful for a lot of applications. In LNLS, there are several labs around the accelerator called “beamlines”, in which scientists from all over Brazil and abroad, working in various fields such as physics, chemistry, biology and materials engineering, use this radiation to collect data that will be analyzed in their research institutions. For more information about the LNLS, click here.
LNLS is the first of its type in the Southern Hemisphere. The main unit is a particle accelerator in the form of a ring, with 93 meters in length, where electrons travel to 99.98% of the speed of light. By doing curves to keep the trajectory along the ring, these electrons emit electromagnetic radiation in the infrared, ultraviolet, X-rays and visible light - it's called "synchrotron radiation." It turns out this radiation is very useful for various researches on the molecular and atomic structure of materials: to study protein structure, the composition of nanomaterials and new materials recently discovered, microcomponents for computers, optical fibers etc.
The equipment employed to transform the the “crude” synchrotron light into radiation proper for research are called "beamlines". In October 2012, 15 of them were installed and running at LNLS. Research institutions throughout Brazil, Latin America, and other countries like South Africa, Norway and Canada use the facilities.

Main ring of LNLS. The storage ring is just below the concrete white protection; it can also be seen the protection of the smaller ring (booster) used during the electron injection procedure that occurs twice a day. One may distinguish some beamlines, like the one comes out of the main ring towards the bottom right of the photo
Source: LNLS site
When Sergio Porto founded the Quantum Electronics Department (DEQ) of IFGW, in early 1970's, he invited several scientists who were abroad to form research groups. Among them were Carlos Rettori, coming from the University of California at Los Angeles (UCLA), and Gaston Barberis, coming from University of Buenos Aires, who founded the group of Magnetic Properties of Materials. At that time, they focused on studies with magnetic resonance. At the end of that decade, the group split up, with the emergence of a new team led by Elion Vargas, who began using magnetic resonance emphasizing on the study of biological materials.
In the mid-1990's, Rettori and Gaston's group merged with José Sanjurjo Antonio (1944-2001), who used the technique of Raman spectroscopy and descended directly from Sergio Porto's group. This increased the range of studies of the group: from the investigation of purely magnetic properties (mostly with magnetic resonance and magnetometry), it has started to include also optical properties.
At that time, there was, however, a great need of the group for the production of the samples used in its research, which were mostly obtained in the form of scientific collaboration with researchers abroad. This hindered investigations as the possibilities of sample supply were limited.
From the early 1990s, the group began to invest in the production of materials and overcame this difficulty. Currently, the permanent faculty staff of GPOMS is formed by Pascoal Pagliuso, Eduardo Granado and Ricardo Urbano. Rettori and Barberis are still part of the group as guest researchers.
Universidade Estadual de Campinas - Instituto de Física Gleb Wataghin
Rua Sérgio Buarque de Holanda, 777
Cidade Universitária, Campinas - SP, 13083-859
Fone +55 19 3521-5297
Fax +55 19 3521-4147