Group of Statistical Physics of Complex Fluids (GFEFC)
- Home
- Research
- DFA - Department of Applied Physics
- Group of Statistical Physics of Complex Fluids (GFEFC)
Fluid is a generic term to talk about something that is not solid – i.e., liquids and gases. Although there is no precise and universally accepted definition, the so-called complex fluids have unique physical and chemical properties, more complex than those of `normal´ fluids. Daily-life examples are several inks (whose different property from the normal fluid is, for example, to drain slightly when applied to a surface, which allows its adhesion and subsequent solidification), adhesives (which "glue"), liquid crystals (whose molecules are capable of partial ordering and can be used to display images, such as in a computer LCD or TV monitor), a shaving cream, shampoo, mayonnaise, detergent, yogurt, and various biological fluids such as blood, milk and even pure water under certain conditions.

There are several phenomena and properties of these fluids that are poorly understood. Therefore, it is important to develop experimental and theoretical studies on the subject. The Complex Fluids Group performs theoretical research, aiming to better understand their properties, the underlying reasons of their peculiar behavior and, in principle, based on this knowledge, to develop new fluids with designed properties.
Although familiar and apparently ordinary, the water can be considered a complex fluid, because its molecules are associated in small groups called "clusters". The water molecules remain associated by links called "hydrogen bonds" (see figure). These links have very short duration and the clusters are not stable, but are continuously being formed and broken up. However, due to the network of hydrogen bonds, from the structural point of view the water looks more like a gel. One of the anomalous properties of water is the fact that its solid phase at low temperatures – which we call `ice´ - is less dense than the liquid phase. Usually, the opposite occurs, i.e., the solid phase is generally denser than the liquid phase. For this reason an ice cube floats in a glass of water. This property, in fact, it is essential to ensure the existence of life in colder climates, as for example, by allowing a fish to survive the winter!
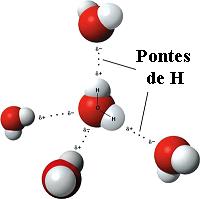
Source: Wikipedia
The complexity of these fluids can arise for several reasons. It occurs, for example, in those fluids composed of very large molecules (macromolecules), which consist of hundreds or thousands of atoms. Examples are the biological fluids, which contain supramolecular aggregates (proteins, micelles, vesicles and so on) immersed in water. The properties of the fluid depend on the shape of the macromolecules and how they interact themselves and with water. Depending on the physico-chemical conditions of the aqueous solution in which they are immersed, the aggregates shape or their mutual interaction can be altered, modifying consequently the macroscopic properties of the fluid.
Another situation arises when different physical states (solid, liquid and gas) coexist microscopically in the fluid. In this case, the fluid is generically called a colloidal suspension. For example, the solid and gaseous parts may coexist in the smoke (consisting of microscopic solid particles dispersed in air). The system formed by gas plus solid particles can be considered as a fluid, as in this case the dispersed solid particles behave as a fluid. Another example: gas and liquid portions occur in very thin foams; gelatins contain solid and liquid pieces; gaseous and liquid parts appear also in aerosols - but, unlike the thin foams, now the gaseous phase is continuous and the liquid phase is dispersed in microscopic droplets. The milk may be regarded as an emulsion, in which proteins, oil droplets and other substances are mixed with water.
The group's research activities focus primarily on investigating the role of charges in determining the properties of ionic complex fluids, so called because they contain free charges (ions). The group wants to know, for example, how the charges distribute and how this distribution defines and changes the thermodynamic properties at the macroscopic and the mesoscopic (i.e., between the microscopic and macroscopic) scales.
A colloidal suspension is nothing more than a mixture, in which the minor component (called solute) occurs as so small particles, such that the system appears macroscopically homogeneous, but there is still no complete dissolution in the major component (called solvent). Examples, as already mentioned earlier, include: milk, mayonnaise, smoke, gelatin, jelly, yogurt.
In an ionic colloidal suspension, in general the solute particles acquire electrical charges only on its surface, at the solute-solvent interface. For example, many proteins contain charged functional groups on its surface (both positive and negative), located at specific positions for the proper biological function of the protein. Some diseases are caused by mutations that change the charge distribution of the normal protein, yielding it to change or lose its function, or even destabilizing its structure, which was conveniently shaped along the natural selection. In addition to specific reasons for each protein, more generally charges on its surface prevent them to collapse. In the case proteins would be completely neutral,they would tend to coalesce, leading to further precipitation.
The charges on the protein surface can also be neutralized by changing the acidity (pH) of the aqueous medium, increasing or decreasing H+ ions concentration in the solution. This happens, for example, when we add vinegar or lemon juice to milk, curdling it. When the acidity of the milk increases, the proteins are destabilized and precipitate, forming the curd.
Another way to destabilize proteins, but that does not involve the electric charges, is by temperature changes, boiled egg being a familiar example. But we can also "boil" an egg mixing it to vinegar or lemon juice. As well as a temperature increase causes destabilization of albumin (the main protein from egg white), an acidity change also destabilizes it. Try to immerse a raw egg (in its shell) inside a cup of vinegar and just see what happens!
Also in paints, composed of microscopic solid particles suspended in water – allowing them to solidify, after they dry - can be stabilized by introducing charges on the surface of the solute particles. The importance of the study of complex fluids is easy to understand in this case of paints, because they must be stable, that is, its solid part can not spontaneously separate from its liquid portion inside the paint can, even under the action of the gravitational forces on the solid particles. The introduction of electric charges on the particles surface prevents precisely that this separation occurs.
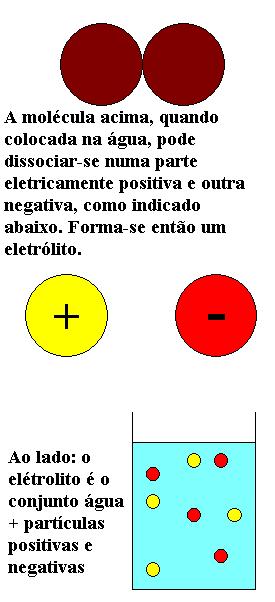
Electrolytes are mixtures of a dissolved substance in another (solute dissolved in solvent) in which the solute is split into smaller oppositely charged particles when immersed in the solvent, as shown in the figure (one says that it dissociates). Thus, the system turns electrically conductive. Polyelectrolytes are electrolytes in which one solute component is generally an ionic polymer containing a large number of charges of the same sign. In turn, the polymers (which may be ionic or not) are chains composed of large sequences of atoms or molecules, called individually monomers. When the monomers are ionic, they can dissociate, and become charged.
For example, the DNA, the macromolecule responsible for the storage and transmission of genetic genetic information and the RNA, responsible for protein synthesis, are examples of polynucleotides (i.e. in this case the monomer is a molecule called nucleotide). Both DNA and RNA are polyelectrolytes, since its constituent monomers contain a phosphate group that becomes negatively charged in aqueous solutions. Yet proteins are polypeptides with hundreds, thousands or millions of amino acids, some of which can be dissociated. But in general, a protein contains both positive as well as negative charge groups. Therefore, typically a protein is not a polyelectrolyte, but is classified as a polyampholyte.
In addition to play a fundamental role in biological systems, the polyelectrolytes have many practical applications. An example is disposable diapers, whose component responsible for the absorption and retention of water is a polyelectrolyte. When the diaper polyelectrolyte is dry, it stays very compact, but when it comes into contact with water, absorbs it, becoming charged (missing ions of opposite sign to the water, called counterions) and becomes a gel of greater volume. The diaper then swells, which is appropriate for its purpose, because it retains the absorbed water. That is, the gel does not act like a sponge, in which the water can be easily removed by application of mechanical pressure. The electrostatic attraction between charges of the polyelectrolyte and of the ions dissolved in water, as they are oppositely charged, prevent this from happening.

Micela
Fonte: Wikipedia
One of the research areas of the group involves ionic phospholipids. Phospholipids are major components of cell membranes. They are said to be ionic when dissociate in aqueous solution and accumulate electric charges on its surface. The individual phospholipid molecules have the particularity that they have a region which tends to repel the water molecules and another that attracts them (thus named amphipathic or amphiphilic molecules). Because of these conflicting properties, the amphiphiles spontaneously tend to self-aggregate, as for example, in the micelle (name given to the structure) shown in the figure, extracted from the Wikipedia. In the cartoon, the portion of the molecule that attracts water is represented by a white ball (called hydrophilic polar head) and the other (inside the micelle) by yellow chains (hydrophobic tails).
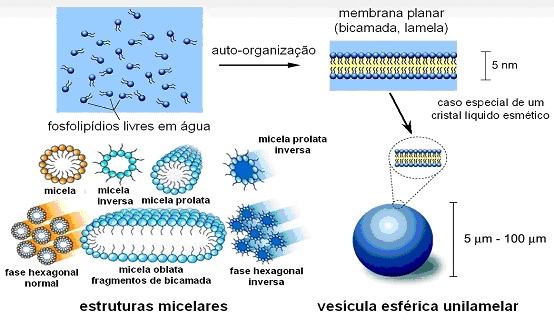
Micelle phase transitions
It turns out that in these systems the supramolecular aggregates may undergo phase transitions, which changes, for example, their geometrical shape, as shown above. Phase transitions are associated with abrupt structural changes, in response to the variation of parameters such as temperature, acidity, presence of other solutes (for example, salt) and so on. Examples of water phase transitions are the transition from the solid (ice) for the liquid and from the liquid to the gaseous state (water vapor) by increasing temperature or decreasing pressure, in which the system density changes discontinuously.
Indeed, already in this "simple" system constituted only by water, we can illustrate the effect of salt addition on the phase transition. In places with a cooler winter, it is common to throw salt on the snow to melt it. This happens because the phase-transition temperature of the solid/liquid mixture of water/salt is lower than that of pure water, which occurs at 0° C.
In the case of phospholipids, one can speak of structural phase transitions: for example, if, at a given temperature, micelles are spherical, by varying the temperature they can suddenly become more elongated, like cigars, or disc-shaped, or even several of these cigars can align to form a larger structure. To this diversity of possible geometrical forms is given the name of polymorphism.
Other types of simpler transitions occur when the hydrophobic tails undergo a change from a more ordered phase to another disordered (called lipid gel-liquid transition) or when the ionic polar heads re-adsorb the counterions initially released in the water. There are several issues not yet understood in these systems, whose theoretical treatment difficulty is enhanced due to the presence of charges in ionic systems.
The actual biological systems are very complicated to allow a full understanding, and at the same time, detailed in physical terms. Therefore, the group researches simplified systems (model systems). For example, in cell membranes, there are several types of phospholipids, besides proteins and other types of molecules. However, we study model membranes composed of only one type of lipid, in order to try to characterize their isolated properties, in the spirit of physical reductionism. That is, the group considers the so-called "biomimetic systems" that mimic biological systems – they are not really biological, but represent a study at the most fundamental level of biological reality. This compartmentalized study has relevance in the study of biology itself, as it can fill gaps in knowledge that exist because of the complexity of the biological systems, which makes prohibitive their detailed study.
The group performs theoretical studies on some of the mentioned types of fluids, using methods of equilibrium statistical physics. The statistical physics is a branch of physics that investigates systems consisting of a large number of interacting elements, establishing a bridge between the microscopic and the macroscopic worlds, the latter allowing measurements. For example, one of the most well known problems in this field is the determination of the macroscopic properties of a gas from the random microscopic motion of the molecules ("elements") that constitute it. It is impossible to describe the simultaneous movement of all molecules of a gas contained in a bottle, but from general assumptions and simplified models, we are able to describe some of its properties.
The group studies use idealized models and methods of statistical physics to try to describe what happens in complex fluids. The emergent behavior of the models can provide important clues about the microscopic mechanisms that produce, for example, associations and organizations of the molecules and other complexities.
The theoretical investigations consist to propose simplified thermostatistical models aimed at understanding the properties of complex fluids, with particular interest in their phase transitions. Among the properties that can be also theoretically investigated and experimentally measured are, for example, the conductivity (ease with which a system conducts electrical current) of the solution of an ionic complex fluid. One can analyze how the conductivity changes by varying the temperature, acidity (pH), addition of salt, etc. Theoretical results can then be compared to the experimental measurements to verify the validity of the real-system description by the theoretical model or the need to introduce other effects not initially incorporated in the model.
The group emerged in late 2005, when Mário Tamashiro was hired by Unicamp, previously working at Institute of Physics, University of São Paulo (IF-USP). He was also a post-doc at other institutions in Brazil (IF-UFRGS) and abroad (UC-Santa Barbara, USA and MPIP-Mainz, Germany). Since then, studies have been done on complex fluids with emphasis on ionic colloidal solutions, polyelectrolytes and biomimetic systems. Research with aggregates of amphiphilic molecules such as phospholipids, are a continuation of participation in a “thematic research project” that comes from 2003, started as a postdoctoral fellow. From 2007, a project was created for the study, by statistical physics, of phase transitions in suspensions of ionic lipids. More recently it has been investigated the ionic distributions at dielectric interfaces, e.g. at air-water interfaces.
Universidade Estadual de Campinas - Instituto de Física Gleb Wataghin
Rua Sérgio Buarque de Holanda, 777
Cidade Universitária, Campinas - SP, 13083-859
Fone +55 19 3521-5297
Fax +55 19 3521-4147